Protecting public health: understanding the importance of cumulative risk assessment of pesticides
Authors (European Food Safety Authority):
Monica del Aguila, Angelo Cafaro, Luis Carrasco Cabrera, Anna F. Castoldi, Nneoma June Katia Chukwubike, Federica Crivellente, Bruno Dujardin, Germán Giner Santonja, Anna Lanzoni, Sara Levorato, Luc Mohimont, Efisio Solazzo
Chemical risk assessment typically focuses on individual chemicals or in a few cases on a combination of chemicals that are expected to occur together. In reality, people are continuously exposed to a wide range of chemicals from various sources, also referred to as unintentional or co-incidental mixtures. The EU Chemicals Strategy for Sustainability[1] sets the framework for assessing the impact of chemical mixtures on human health and the environment.
Cumulative risk assessment (CRA) of pesticides is a scientific methodology used to estimate combined health risks associated with exposure to multiple pesticides. The CRA process involves several steps, including the identification of relevant pesticides, the evaluation of their toxicity in the most critical organs/systems, the estimation of dietary exposure levels and the assessment of the potential health risks for different population groups.
What are the main EU policies on CRA of pesticides?
EU Regulation 1107/2009 (placing of plant protection products in the market) and EU Regulation 396/2005 (on maximum residue levels in food and feed) require cumulative and synergistic effects of residues of plant protection products to be taken into consideration.
The European Food Safety Authority (EFSA) and the European Commission developed an action plan for the gradual implementation of CRA for pesticides by EFSA and Member States from 2022 onwards[2]. This plan provides that all toxicological effects of pesticides of relevance for CRA will have been identified by 2030 and cumulative assessment groups (CAG) in the respective organs and systems will have been established. This action plan on the implementation of CRA of pesticides and their residues is complemented by the RACEMiC (Risk Assessment of Combined Exposure to Multiple Chemicals) roadmap[3], which aims to implement similar approaches across all of EFSA’s regulatory domains. Within this context, EFSA will further explore collaborations and synergies with other external partners, including the Partnership for the Assessment of Risks from Chemicals (PARC).
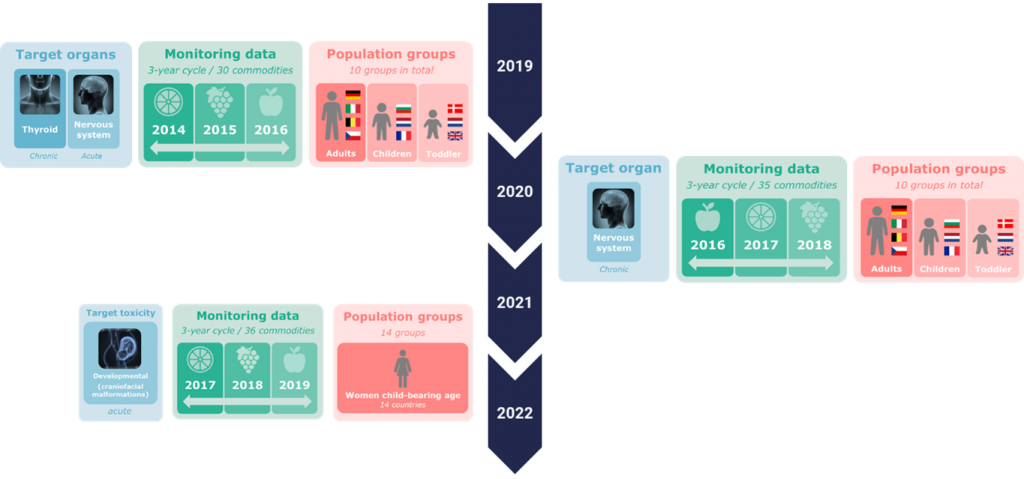
So far retrospective CRAs have been performed: CRA for chronic effects on the thyroid[4], CRA for acute[5] and chronic[6] effects on the nervous system, and CRA for acute craniofacial alterations[7] (figure 1). CRAs for liver, kidney and reproductive toxicity are ongoing. Retrospective assessments will need to be repeated on a regular basis to account for changes in the exposure patterns and possible updates of the CAGs.
—
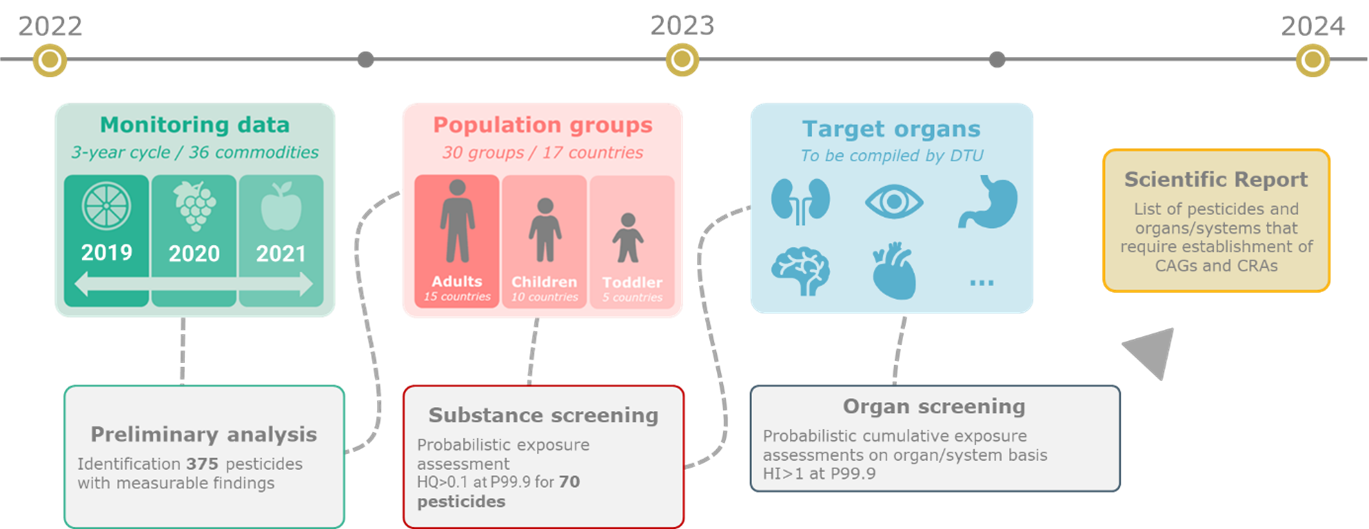
To ensure an optimal use of resources, EFSA developed a prioritisation method which allowed the identification of pesticides and organs/systems of relevance for dietary CRA (figure 2). The prioritisation method is published on a EFSA scientific report[8]. On the longer term, the implementation of the prioritisation method is intended to be repeated every three years. Examples of CRAs to be performed in the future are related to the reproductive (male and female) toxicity, developmental toxicity, or developmental neurotoxicity.
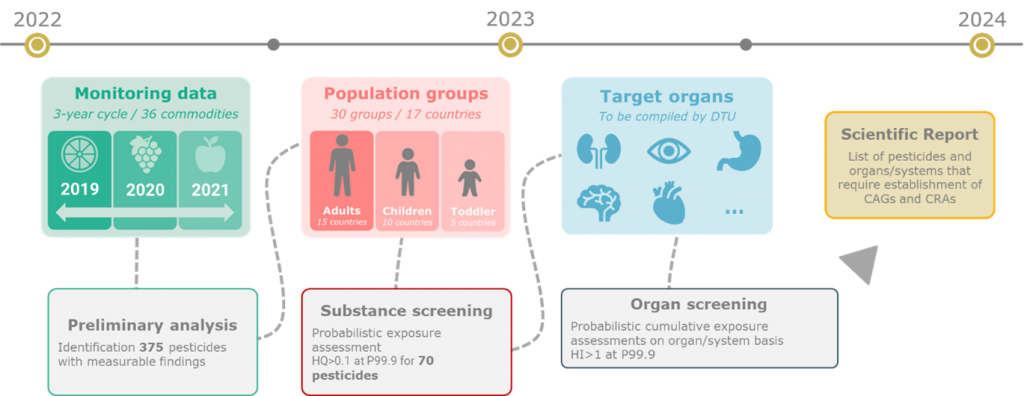
—
How is a retrospective CRA of pesticide residues performed by EFSA?
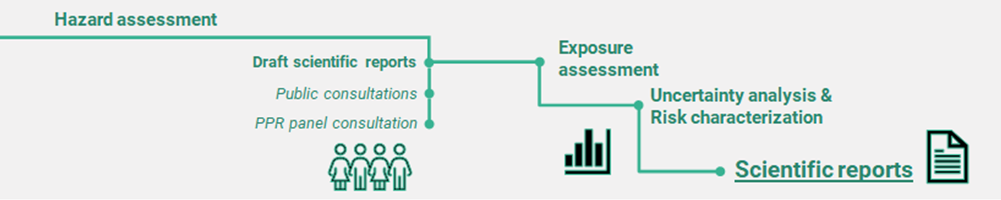
A retrospective CRA consists of a sequence of steps[9] which are summarised as follows (figure 3):
- – Hazard identification: identification and definition of the specific effects of relevance for CRA
- – Hazard characterisation: characterisation of the potency of the active substances and establishment of CAGs
- – Public consultation and consultation of the Panel on Plant Protection Products and their Residues on the draft scientific reports
- – Cumulative exposure assessment performed by probabilistic modelling conducted with the MCRA[10] platform, including several sensitivity analyses
- – Uncertainty analysis using expert knowledge elicitation and risk characterisation
- – Finalisation of scientific reports
—
How are the results of the retrospective CRA used by risk managers?
During the Standing Committee on Plants, Animals, Food and Feed (SCoPAFF) of 11–12 June 2015, Member States agreed on the use of the combined margin of exposure (MOET, also known as Total Margin of Exposure) concept as the mode of calculation and expression of cumulative risks.
Furthermore, during the SCoPAFF of 18–19 September 2018, Member States agreed on a MOET of 100 at the 99.9th percentile of exposure as a general threshold for regulatory consideration and as an indicative target of safety in consistency with the safety margin currently used for establishing toxicological reference values (i.e. assuming a factor of 10 for interspecies variability and a factor of 10 for intraspecies variability). In case the respective MOET is below 100, regulatory consideration would be required.
Cumulative exposure did not exceed the threshold for regulatory consideration for any of the population groups considered during the retrospective CRAs performed so far.
—
Disclaimer: The views or positions expressed in this publication do not necessarily represent in legal terms the official position of the European Food Safety Authority (EFSA). EFSA assumes no responsibility or liability for any errors or inaccuracies that may appear.
—
[2] https://food.ec.europa.eu/system/files/2021-03/pesticides_mrl_cum-risk-ass_action-plan.pdf
[3] https://doi.org/10.2903/sp.efsa.2022.EN-7555
[4] https://www.efsa.europa.eu/en/efsajournal/pub/6088
[5] https://www.efsa.europa.eu/en/efsajournal/pub/6087
[6] https://www.efsa.europa.eu/en/efsajournal/pub/6392
[7] https://www.efsa.europa.eu/en/efsajournal/pub/7550
[8] https://www.efsa.europa.eu/en/efsajournal/pub/8554
[9] A more detailed description of the CRA steps is provided in Table 1 of this document: https://www.efsa.europa.eu/sites/default/files/documents/art36/gpefsaprev202303/call-for-proposal-cumulative-risk-assessment.pdf
Latest Articles
Towards holistic, AI-driven emerging risk assessment: catching stakeholders’ needs in Living Labs

Protecting public health: understanding the importance of cumulative risk assessment of pesticides

SCAR consultation workshop on Sustainable Food Systems: highlights from EU FOOD SAFETY PLATFORM

Pioneering advances in the EU Food Safety System: highlights from the first EU Food Safety Forum
Food4Future_cz

New Tools for Preventing Harmful Bacteria in Ready-to-Eat Foods



